Source:https://www.newtechaqua.eu/meagre-and-greater-amberjack-reproduction-in-captivity-results-of-the-newtechaqua-project/
Meagre (Argyrosomus regius) and greater amberjack (Seriola dumerili) are emerging species with great interest, but a slowly increasing production in the Mediterranean aquaculture. The flesh of these two large-sized and fast-growing fish is well appreciated by consumers, making them excellent candidates for the diversification and growth of the European market and suitable for being processed into a large number of value-added products.
When reared in captivity, though, both species exhibit reproductive dysfunctions, which result in a failure to undergo complete gametogenesis (greater amberjack), maturation (meagre), or spawning (both species).
The observed impairment of the reproductive axis is caused by the absence of natural-like environmental conditions, stress induced by captive conditions (i.e. human activities, handling), or a combination of both (Mylonas et al., 2010; Zohar, 1989). Therefore, the acquisition of knowledge on the physiology of the reproductive cycle and the development of methods to enhance reproductive performance in these two economically important species, are vital for obtaining good quality fertilized eggs and closing their reproductive cycle in captivity.

The studies conducted by the Hellenic Center for Marine Research (HCMR) in the frame of the NewTechAqua project, included the assessment of:
a) the effect of temperature and salinity manipulations on reproductive maturation, spawning success and egg/sperm quality in meagre and
b) the effect of moving greater amberjack broodstocks from cages to large-volume tanks for achieving spontaneous spawning.
Figure 1. Dr. CC Mylonas (front) with team members from the Hellenic Centre for Marine Research (HCMR) during a meagre reproduction experiment.
a) Experiments on meagre reproduction
1.1. Effect of temperature manipulations on reproductive maturation, spawning success and egg/sperm quality in meagre
The annual reproductive cycle in fishes in the temperate or higher latitudes is controlled by environmental cues, mainly photoperiod and temperature, with photoperiod being the principal environmental regulator of the process of gametogenesis, and temperature acting as a secondary cue, being more important during oocyte maturation, spermiation and spawning. In the temperate zone, where progressive variations occur seasonally, temperature may work in synergy with photoperiod. These changes are reliable signals that fish can use to synchronize their biological rhythms to reproduce during the most favorable moment of the year, and exposure to abnormal temperature profiles may alter the amount and quality of the gametes (Durant et al., 2007). This information is of practical importance for the aquaculture industry, which usually simulates both photic and thermal conditions to achieve seasonal reproductive development and production of eggs (Bromage et al., 2001; Migaud et al., 2006) as well as off-season spawning (Carrillo et al., 1989). As a result, significant costs for infrastructure and energy are incurred to heat and cool the water in order to provide the thermal cycling usually encountered in nature.
To verify the possibility of maintaining broodstocks under simulated natural photoperiod, but relatively constant borehole water temperatures, which would save in infrastructure and energy, we examined a) the effect of a constant thermal regime (CoT) compared to an attenuated seasonal thermal regime (SeasT) in the process of gametogenesis, sperm production and quality and b) the response to GnRHa administration in terms of spawning kinetics and egg production.

Figure 2. Broodstock reproductive evaluation of female meagre (Argyrosomus regius) through collection of ovarian biopsies, using an endometrial catheter (left) and estimation of the mean diameter of the largest vitellogenic oocytes (right).
Two mixed-sex broodstocks (N = 14-16) were maintained in 15-m3 rectangular tanks in recirculating aquaculture systems (RAS) under simulated natural photoperiod and supplied with borehole seawater, exposed to either a simulated “attenuated” seasonal temperature profile (SeasT group; 16.4 – 19.6ºC) previously established for meagre maturation (Mylonas et al., 2015; Mylonas et al., 2016), or a constant temperature profile (CoT group; 19.4 ± 0.6ºC) resulting from the use of a typical borehole seawater source used in commercial hatcheries in the Mediterranean. Four females per group were selected at the expected spawning period (day 0) based on their ovarian maturity stage evaluation (mean oocyte diameter of the most advanced vitellogenic oocytes >500 μm), and were injected with gonadotropin releasing hormone agonist (GnRHa) at an effective dose of 14.3±0.2 μg GnRHa kg-1 BW (Mylonas et al., 2016). After treatment, females were placed individually in eight separate 5-m3 rectangular flow-through tanks supplied with aerated borehole seawater at 19.7±0.4ºC under simulated natural photoperiodic conditions. The four selected males from each thermal group (SeasT or CoT) were treated with a GnRHa implant at an effective dose of 48.0±2.7 μg GnRHa kg-1 BW (Mylonas et al., 2013b) constructed with [Ethylene-Vinyl Acetate]-copolymer and were also placed in the 5-m3 tanks with the females from their respective temperature profile group. This way, four couples per temperature group were formed and induced to spawn. All GnRHa-treated fish were sampled weekly for the following four weeks. Females were injected with the same dose of a GnRHa injection at each of the following three weeks (days 7, 14, 21). Males were treated with the same GnRHa implant of the same dose once again at the beginning of the third week (day 14). At each sampling, biopsies and sperm were collected when it was possible, mean oocyte diameters of the largest vitellogenic oocytes were calculated and the spermiation index was determined objectively on a scale from S0 to S3 as follows: S0 = no milt released, S1 = only a drop of milt released after multiple stripping attempts, S2 = release of milt after the first strip attempt, and S3 = copious amount of sperm released with very little pressure. On day 28 the experiment was completed and all fish were transferred back to their original tanks.
In the SeasT females, spawning commenced 2 days after the first GnRHa injection and a second spawn was obtained also the following day (Fig. 3). On the contrary, not all females from the CoT group spawned after the first GnRHa injection, and spawning was very erratic. Fecundity was also many folds higher in the SeasT females at the first spawning induction. In subsequent weekly ovarian evaluations, no significant differences in mean diameters of the largest vitellogenic oocytes were observed between the SeasT and CoT females, but a trend of lower values was noted in the CoT females (Fig. 4). Spawning after the subsequent GnRHa injections was not as consistent in the SeasT females as it was after the first GnRHa injection, while in the CoT females it seemed to occur in more synchrony than before (Fig. 3).
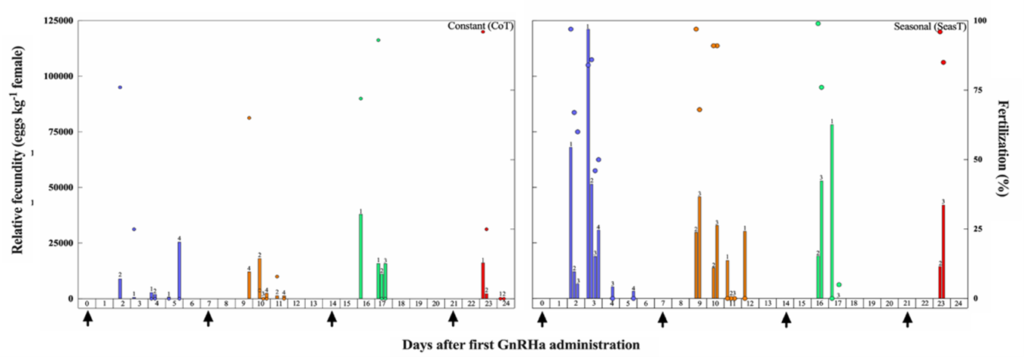
Figure 3. Daily relative fecundity (bars, eggs kg-1 female) and fertilization success (circles, %) of individual meagre females (Argyrosomus regius) induced to spawn with multiple GnRHa injections (n = 4, arrows) after exposure to either a Constant (CoT) or attenuated Seasonal (SeasT) thermal regime (n = 4, per thermal regime). Different colors correspond to spawns after consecutive GnRHa injections. Numbers above the bars indicate the ID of the individual female, in order to show which female produced each spawn.
Regarding the males, the administration of two GnRHa implants improved steadily the spermiation index of individuals from the SeasT group, and milt could be collected and analyzed from almost all males at all sampling times (Fig. 2B). A similar, but much less pronounced effect was observed in GnRHa treated males from the CoT group. This demonstrates that the fish had the capacity to respond to the GnRHa stimulation -and the expected increase in plasma Luteinizing Hormone (LH) and sex steroid production (Mylonas et al., 2017)- but their initial stage of reproductive development was such that spermiation and releasable sperm production was reduced.
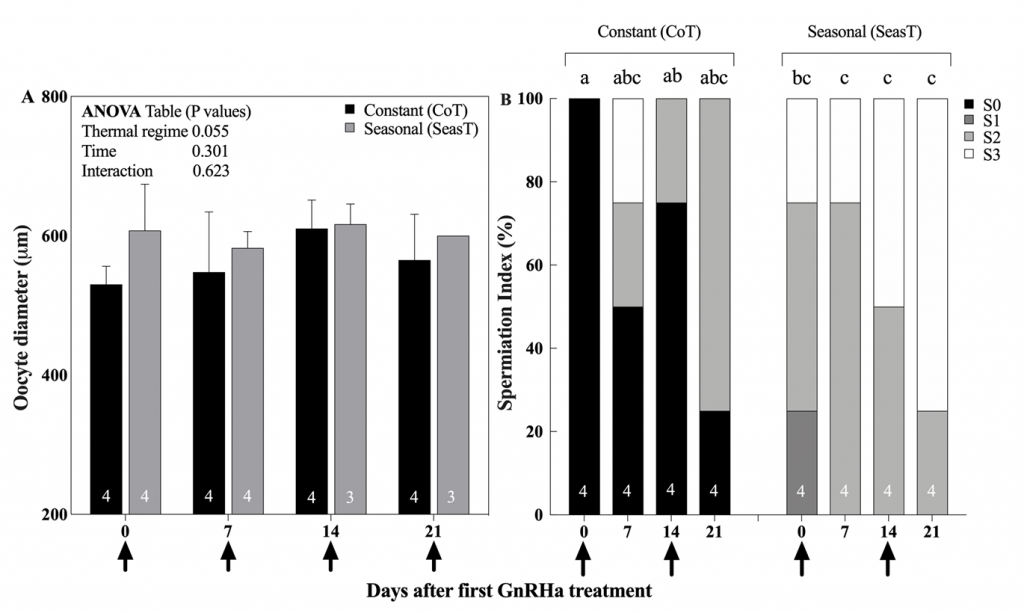
Figure 4. Mean (± SEM) diameter of the largest vitellogenic oocytes from ovarian biopsies (A) and percentage (%) males at different spermiation index stages (B) of meagre (Argyrosomus regius) breeders exposed to a Constant (CoT, n=4) or attenuated Seasonal (SeasT, n=4) thermal regime, and selected for spawning induction. Arrows on the x-axis indicate the time of GnRHa administration (injection in females, implantation in males). Numbers inside the bars indicate the N value of the means. No significant differences were observed during the spawning induction experiment in oocyte diameters, but significant differences in spermiation index among thermal regime/sample time combinations were observed (Friedman’s test, Dunn’s post hoc, P≤0.05), indicated by different lowercase letters above the bars.
In conclusion, the exposure throughout the year to relatively constant water temperatures typical of borehole water in the Mediterranean, did not prevent gametogenesis in either males or females. However, the results underlined the necessity of at least a winter thermal profile for the proper progression and completion of the gametogenic process. Therefore, maintaining broodstocks on seasonal photoperiod but constant borehole water temperature throughout the year cannot be utilized as a cost-effective method for aquaculture production for meagre.
1.2 Effect of salinity manipulations on reproductive maturation, spawning success and egg/sperm quality in meagre
Meagre is an anadromous marine fish that enters estuaries and large rivers for spawning, thus it is expected that lower salinity is a natural environmental condition at this time of the reproductive cycle. In commercial Mediterranean aquaculture, meagre broodstocks (as all other fish species) are maintained in full salinity water throughout the year, but fail to undergo spontaneous maturation, ovulation and spawning in aquaculture, and require hormonal induction of spawning. Although in euryhaline species, such as the meagre, salinity may be an environmental factor influencing the reproductive physiology (Bromage et al., 2001), as fish migrate from seawater to water bodies with lower salinity at the onset of the reproductive season, up to date, there are no reports on whether meagre need to enter low salinity waters to complete oocyte maturation, and the precise areas where spawning occurs are still unknown.
In this experiment, the broodstock was maintained in full salinity (34‰) between spawning seasons, and was exposed to a natural simulated photoperiod and a simulated “attenuated” seasonal temperature profile previously established for meagre maturation during the current spawning season (De Mello et al., 2021; Mylonas et al., 2016). Four experimental groups were formed in duplicates, and exposed to different salinities for a month (positive Control with 34‰ salinity, SW34‰, SW20‰, and SW12‰)
Females from the positive Control group (n=2), were injected with GnRHa at an effective dose of 16.7 ± 1.7 μg GnRHa kg-1 BW (Mylonas et al., 2016). The females from the SW34‰, SW20 and SW12 were transferred without a hormonal administration and were allowed to spawn spontaneously. The males (n = 16) from all the experimental groups were treated with a GnRHa implant at an effective dose of 65.8 ± 2.8 μg GnRHa kg-1 BW (Mylonas et al., 2013a) embedded in [Ethylene-Vinyl Acetate]-copolymer.
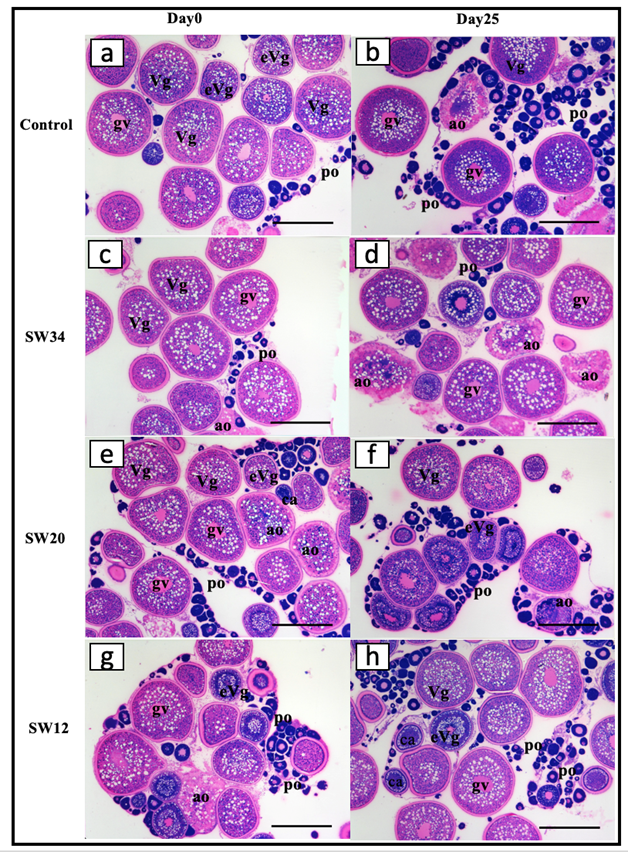
Figure 5. Histological sections of meagre (Argyrosomus regius) ovarian biopsies during the broodstock evaluation for the formation of the experimental groups on day 0 (a, c, e and g) and on day 25 after exposed to different salinity regimes (b, d, f and h). po – primary oocytes, Vg – Vitellogenic oocyte, ao -apoptotic oocytes, ca – cortical alveoli, gv – germinal vesicle. Bars represent 500 μm.
On day 0 of the experiment the females selected for the experiment (n = 8) were at late vitellogenesis (Vg). The mean (±SD) diameter of the largest Vg oocytes was at 578 ± 7 μm which did not change along the experiment in relation to environmental salinity. However, the number of the late Vg oocytes in the positive Control group decreased on day 25 compared to day 0 of the experiment concomitantly with an increase in the number of primary oocytes (PO) (Fig. 5a, b). This was expected after spawning, since these fish spawned many times after GnRHa administration, therefore, the pool of late vitellogenic oocytes was reduced, and some oocytes begun the process of apoptosis once the GnRHa stimulus subsided. On day 25 an increase in follicular atresia of the ovarian biopsies was observed in the SW34 group (Fig. 5c, d). These females, which were maintained under the same salinity (SW34) as the positive Control group, but were not given hormonal treatment, exhibited ovaries containing a larger number of late vitellogenic oocytes, extensive atresia, and a negligible number of early stage vitellogenic oocytes, which confirmed the common reproductive dysfunction observed in meagre broodstock maintained in seawater during the reproductive season, that is the failure of oocyte maturation and ovulation (Duncan et al., 2012, 2013; Fernandez-Palacios et al., 2014). On the other hand, on the same day, both SW20 and SW12 ovarian biopsies were in better condition, since less follicular atresia was observed compared to the start of the experiment (Fig. 5e-h). This could lead to the cautious assumption that the reduction of salinity functioned as a stimulus to support and protract in time the reproductive efforts of females during the present study.
Although the aim of this study was the investigation of the effect of salinity and its potential in inducing spontaneous spawning in meagre females, some differences were found also in males as their exposure to water with a lower salinity seemed to be beneficial on the gonadal maturation and the capacity of males to release milt (Fig. 6). On day 0, before GnRHa implantation, males presented a variable maturation state, whereas at the end of the experiment at day 25, 50% and 66% of males from groups SW12 and SW20, respectively, exhibited an ameliorated spermiation condition releasing a copious amount of milt after gentle pressure on the abdomen, and a general decrease in the numbers of individuals with no sperm release. On the opposite, 100% of the males from the positive Control group did not release any sperm when stripping was applied.
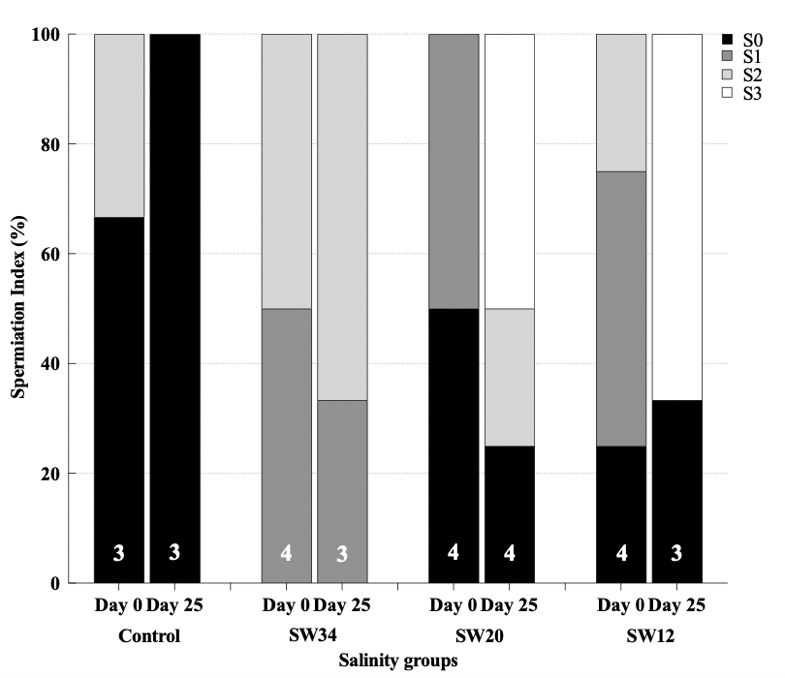
Figure 6. Percentage (%) of meagre males (Argyrosomus regius) at different spermiation condition on day 0 and on day 25 after breeders were exposed to different salinity regimes. All the males were administered GnRHa implants on day 0. Numbers inside the bars indicate the N value of the means.
The findings of this study suggest that the exposure to lower salinity during the expected spawning season did not result in spontaneous spawning in meagre, though it did seem to enhance the vitellogenic process, prevent follicular atresia of post vitellogenic oocytes in females and enhance spermiation condition in the males. However, this methods cannot be recommended to the industry as a method to improve reproductive performance in meagre.
b) Experiment on greater amberjack reproduction – effect of moving the broodstock from cages to large-volume tanks for achieving natural spawning
Inappropriate environmental conditions in captivity may cause reproductive dysfunctions in fish (Mylonas et al., 2010). In the Mediterranean, it has been demonstrated that when maintained in tanks, greater amberjack rarely complete oogenesis and spermatogenesis, thus failing to spawn and produce fertilized eggs (Fakriadis et al., 2020b). While males presented a premature cessation of spermatogenic activity, much earlier than fish in the wild, together with an atypical increase of apoptosis, females displayed large-scale atresia of vitellogenic oocytes when kept in tanks (R. Zupa et al., 2017a; Rosa Zupa et al., 2017b)


Figure 7. Overview of the Argosaronikos Fishfarms SA sea cages (left) and tanks (right) used during the spawning experiment in Salamina, Greece.
Up to date, the most effective method to obtain good quality eggs from Mediterranean greater amberjack (Seriola dumerili) has been to a) maintain breeders in sea cages during the year where they undergo full gametogenesis and then b) move them to land-based tanks after GnRHa induction of spawning (Fakriadis et al., 2019, 2020). In order to reduce the reliance of the industry on the usage of hormonal treatments for spawning induction, and potentially also improve egg quality parameters, large diameter tanks were tested at the aquaculture facilities of Argosaronikos Fishfarms SA (Salamina, Greece) (Fig. 7), to verify the possibility that large volumes would allow spontaneous maturation and spawning of fully vitellogenic females and spermiating males.
In our study, in the first tank fish were given GnRHa in a controlled release implant (Induced, six males of mean ± SD weight of 22.1 ± 1.6 kg, and four females of 25.0 ± 2.03 kg). In the second tank (Spontaneous, five males of 19.0 ± 0.56 kg and four females of 29.7 ± 0.92 kg), fish did not receive any hormonal treatment.
Females treated with GnRHa spawned 12 times, out of which 8 were the following days after hormonal therapy (Fig. 8). On the contrary, the Spontaneous group spawned less frequently for a total of 6 times. The mean spawning batch relative fecundity (t-test, P = 0.009) (Fig. 9A) and fertilization success (t-test, P = 0.001) (Fig. 9B) of the Spontaneous group were higher than the Induced group.
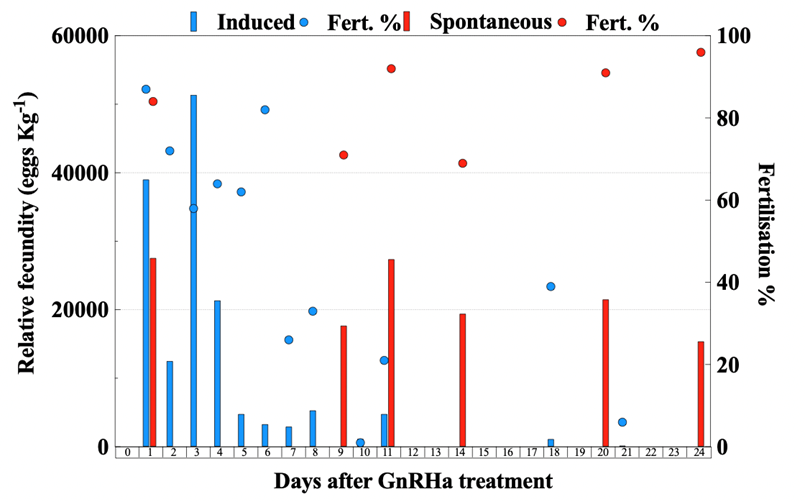
Figure 8. Daily relative fecundity (bar, eggs Kg-1 female) and fertilization success (circle, %) of greater amberjack (Seriola dumerilii) implanted with GnRHa (blue) and not treated (red).
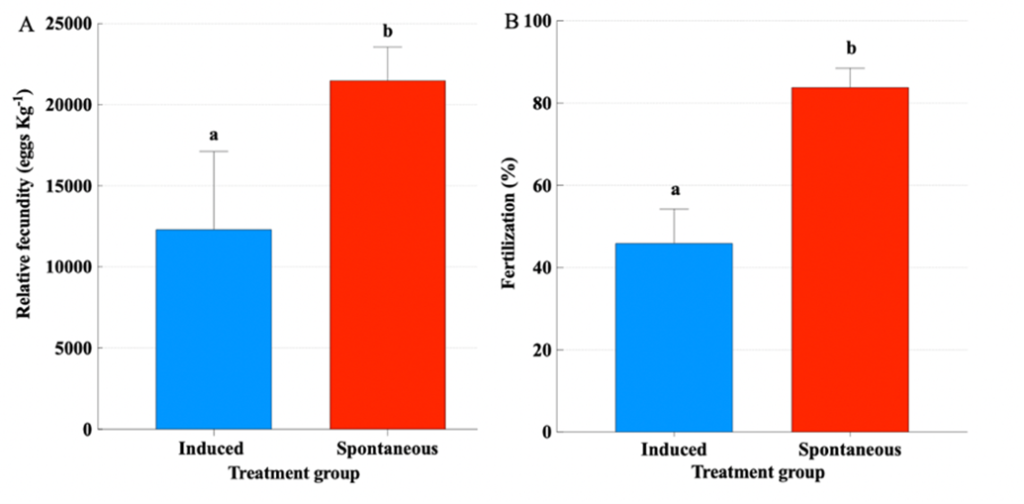
Figure 9. A) Mean (± SEM) total relative fecundity (eggs kg-1 female) of greater amberjack (Seriola dumerili) treated with GnRHa or without hormonal treatment (t-test, P ≤ 0.05). B) Mean (± SEM) fertilization success (%) of greater amberjack. When present, lowercase letters above the means indicate significant differences between groups. Numbers inside the bars indicate the N value of the means.
Therefore, providing greater amberjack with large-volume tanks, allowed spontaneous and consistent spawning with excellent fecundity, fertilization and embryo survival, which were higher than the values obtained from GnRHa-induced breeders (Fig. 9). Sperm quality parameters after 24 days were similar between GnRHa-treated and non-treated males, indicating that, also in males, allowing greater amberjack to spawn in large tanks can enhance reproductive performance and deliver a potential tool for the production of high-quality gametes for hatchery production.
References
Bromage, N., Porter, M., Randall, C., 2001. The environmental regulation of maturation in farmed finfish with special reference to the role of photoperiod and melatonin, Reproductive Biotechnology in Finfish Aquaculture. Woodhead Publishing Limited. https://doi.org/10.1016/B978-0-444-50913-0.50008-4
Carrillo, M., Bromage, N., Zanuy, S., Serrano, R., Prat, F., 1989. The effect of modifications in photoperiod on spawning time, ovarian development and egg quality in the sea bass (Dicentrarchus labrax L.). Aquaculture 81, 351–365. https://doi.org/10.1016/0044-8486(89)90159-2
De Mello, P., Lancerotto, S., Fakriadis, I., Tsoukali, P., Papadaki, M., & Mylonas, C., 2021. The importance of thermoperiod for proper gametogenesis and successful egg and sperm production in meagre (Argyrosomus regius) breeders in aquaculture. Mediterr. Mar. Sci. 22, 218–231.
Duncan, N., Estévez, A., Porta, J., Carazo, I., Norambuena, F., Aguilera, C., Gairin, I., Bucci, F., Valles, R., Mylonas, C., C., 2012. Reproductive development , GnRHa-induced spawning and egg quality of wild meagre ( Argyrosomus regius ) acclimatised to captivity. Fish Physhiology Biochem. 38, 1273–1286. https://doi.org/10.1007/s10695-012-9615-3
Duncan, N.J., Estévez, A., De, U., Gran, P. De, Gairin, I., Roo, J., Schuchardt, D., 2013. Aquaculture production of meagre ( Argyrosomus regius ): hatchery techniques , ongrowing and market, Advances in aquaculture hatchery technology. Woodhead Publishing Limited. https://doi.org/10.1533/9780857097460.3.519
Durant, J.M., Hjermann, D., Ottersen, G., Stenseth, N.C., 2007. Climate and the match or mismatch between predator requirements and resource availability. Clim. Res. 33, 271–283. https://doi.org/10.3354/cr033271
Fakriadis, I., Lisi, F., Sigelaki, I., Papadaki, M., Mylonas, C.C., 2019. Spawning kinetics and egg/larval quality of greater amberjack (Seriola dumerili)in response to multiple GnRHa injections or implants. Gen. Comp. Endocrinol. 279, 78–87. https://doi.org/10.1016/j.ygcen.2018.12.007
Fakriadis, I., Sigelaki, I., Papadaki, M., Papandroulakis, N., 2020b. Control of reproduction of greater amberjack Seriola dumerili reared in aquaculture facilities. Aquaculture 519, 734880. https://doi.org/10.1016/j.aquaculture.2019.734880
Fakriadis, I., Zanatta, E.M., Fleck, R., Dos, P., Lorena, D., Mateo, S., Papadaki, M., Mylonas, C.C., 2020a. Endocrine regulation of long-term enhancement of spermiation in meagre (Argyrosomus regius) with GnRHa controlled-delivery systems. Gen. Comp. Endocrinol. 113549. https://doi.org/10.1016/j.ygcen.2020.113549
Fernandez-Palacios, H., Schuchardt, D., Roo, J., Izquierdo, M., Hernandez-Cruz, C., Duncan, N., 2014. Dose-dependent effect of a single GnRHa injection on the spawning of meagre (Argyrosomus regius) broodstock reared in captivity. Spanish J. Agric. Res. 12, 1038–1048. https://doi.org/10.5424/sjar/2014124-6276
Migaud, H., Wang, N., Gardeur, J.N., Fontaine, P., 2006. Influence of photoperiod on reproductive performances in Eurasian perch Perca fluviatilis. Aquaculture 252, 385–393. https://doi.org/10.1016/j.aquaculture.2005.07.029
Mylonas, C.C., Duncan, N.J., Asturiano, J.F., 2017. Hormonal manipulations for the enhancement of sperm production in cultured fi sh and evaluation of sperm quality. Aquaculture 472, 21–44. https://doi.org/10.1016/j.aquaculture.2016.04.021
Mylonas, C.C., Fatira, E., Karkut, P., Papadaki, M., Sigelaki, I., Duncan, N.J., 2015. Reproduction of hatchery-produced meagre Argyrosomus regius in captivity III. Comparison between GnRHa implants and injections on spawning kinetics and egg/larval performance parameters. Aquaculture 448, 44–53. https://doi.org/10.1016/j.aquaculture.2015.05.036
Mylonas, C.C., Fostier, A., Zanuy, S., 2010. General and Comparative Endocrinology Broodstock management and hormonal manipulations of fish reproduction. Gen. Comp. Endocrinol. 165, 516–534. https://doi.org/10.1016/j.ygcen.2009.03.007
Mylonas, C.C., Mitrizakis, N., Papadaki, M., Sigelaki, I., 2013. Reproduction of hatchery-produced meagre Argyrosomus regius in captivity I . Description of the annual reproductive cycle. Aquaculture 414–415, 309–317. https://doi.org/10.1016/j.aquaculture.2013.09.009
Mylonas, C.C., Salone, S., Biglino, T., Mello, P.H. De, Fakriadis, I., Sigelaki, I., Duncan, N., 2016. Enhancement of oogenesis / spermatogenesis in meagre Argyrosomus regius using a combination of temperature control and GnRHa treatments. Aquaculture 464, 323–330. https://doi.org/10.1016/j.aquaculture.2016.07.006
Zohar, Y., 1989. Endocrinology and fish farming: Aspects in reproduction, growth, and smoltification. Fish Physiol. Biochem. 7, 395–405. https://doi.org/10.1007/BF00004734
Zupa, R., Fauvel, C., Mylonas, C.C., Pousis, C., Santamaria, N., Papadaki, Μ., Fakriadis, I., Cicirelli, V., Mangano, S., Passantino, L., Lacalandra, G.M., Corriero, A., 2017. Rearing in captivity affects spermatogenesis and sperm quality in greater amberjack, (Risso, 1810). J. Anim. Sci. 95, 4085. https://doi.org/10.2527/jas2017.1708
Zupa, Rosa, Rodrõâguez, C., Mylonas, C.C., Rosenfeld, H., Fakriadis, I., Papadaki, M., Peârez, J.A., Pousis, C., Basilone, G., Corriero, A., 2017. Comparative study of reproductive development in wild and captive-reared greater amberjack seriola dumerili (Risso, 1810). PLoS One 12, 1–28. https://doi.org/10.1371/journal.pone.0169645




