Phyto- and zooplankton production
Key research areas
Husbandry methods, Species diversification, Exploitation of local diatoms for biotechnological applications
People involved

Nikos Papandroulakis
Research Director
2810337733
npap@hcmr.gr
| Research Directions |
| Services |
|
|


The Institute operates a live food chain production facility for microalgae, rotifers, and Artemia.
Starting from pre-cultures, microalgae are produced in classical plastic sacks or in vertical-tubular and flatbed photobioreactors at high density cultures (200-300 million cells ml-1). Currently, two flatbed reactors of 1200-L and a vertical-tubular one of 1800-L (dark phase included) are in operation.
The photobioreactors use natural light, taking advantage of the long days and sunny conditions in Crete. A local strain of marine Chlorella sp. is mostly used although Tetraselmis sp. and T-Iso are also cultured.
Zooplankton, such as rotifers (Brachionus sp.) and Artemia are produced on an industrial scale by means of standard techniques. Two rooms with controlled water temperature are used, with 12 tanks for the rotifers (8×1.6 m3 for culture and 4×0.8 m3 for enrichment) and 10 tanks for the Artemia (3×0.5 m3 for the hatching of cysts and 7×0.8 m3 for the culture of the instars).
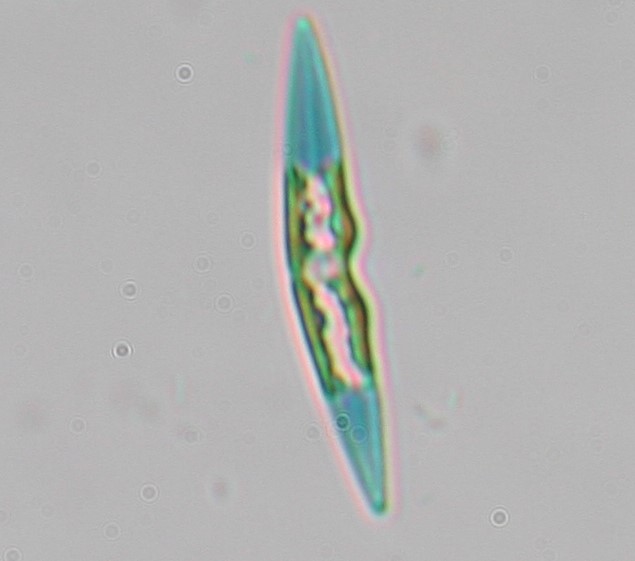
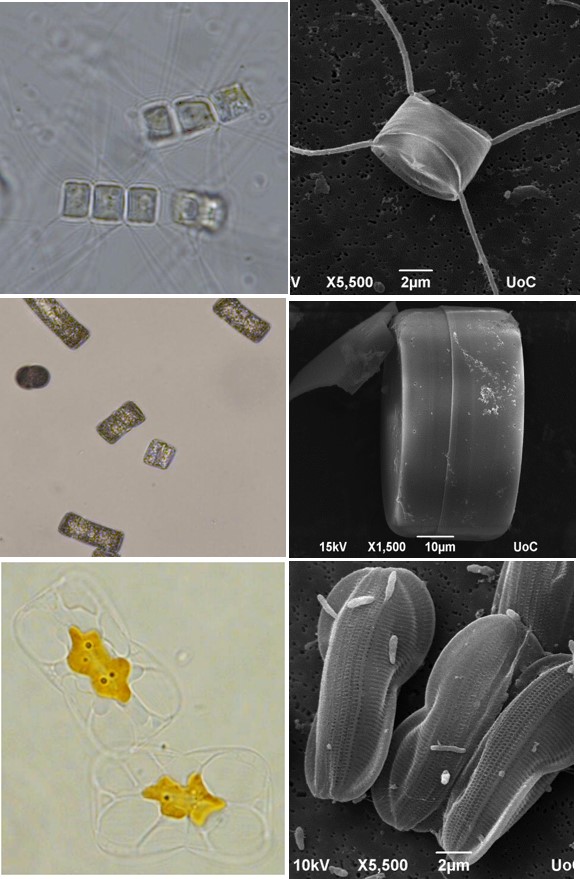
Another unit implements research on diatoms. These are prominent unicellular eukaryotic algae responsible for a fourth of the earth’s oxygen, playing a pivotal role in the marine food web and geochemical cycles of carbon and silica. Diatoms present promising applications in a wealth of industries and technologies including nanotechnology, biofuels, environmental assessment, pharmaceutics, bioremediation and aquaculture. Specifically, diatoms biosynthesize a wide range of lipids including omega-3 polyunsaturated fatty acids (PUFAs) which are essential nutrients required in aquaculture feeds. Some of the research objectives of this unit include
- RNA silencing and acclimation of diatoms to stress conditions increasing lipids biosynthesis
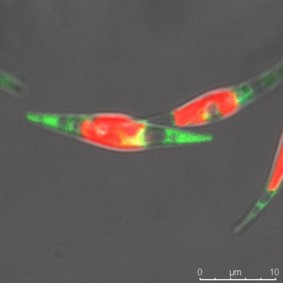
- Characterization of marennine and highly branched isoprenoid biosynthesis pathways in the bleu diatom Haslea ostrearia
- Exploitation of local diatom diversity for biotechnological applications
Previous/past research programs in diatoms:
1. RNA silencing and acclimation of diatoms to stress conditions increasing lipids biosynthesis
Nitrogen is one of the most frequently limited nutrient for diatom growth in their natural environment. Diatom acclimatory response to nitrogen starvation involved complex genetic and metabolic rearrangements leading to a substantial increase in lipid accumulation. RNA silencing is a mechanism of regulation of gene expression mediated by small RNAs. Recent studies in the model diatom species Phaeodactylum tricornutum have shown that RNA silencing plays an important role in diatom acclimatory response to nitrogen starvation. We are conducting a reverse genetic approach employing mutagenesis, omics and biochemical analysis to characterize the molecular components and mechanisms of RNA silencing in P. tricornutum.
(RADIO-ELIDEK 483)
2. Characterization of marennine and highly branched isoprenoid biosynthesis pathways in the bleu diatom Haslea ostrearia
The diatom Haslea ostrearia produces a blue pigment called marennine with applications as prophylactic agent in aquaculture. In addition, H. ostrearia produces a range of specific highly branched isoprenoids with application in biofuels and paleo-environmental studies. Haslea sp. from various locations and with contrasting marennine biosynthesis capacities are cultured in laboratory. Comparative omics analyses are carried out to pinpoint candidate genes involved in marennine and HBI biosynthesis. In parallel, P. tricornutum is employed as a cell chassis to express H. ostrearia marennine and HBI biosynthesis gene candidates for their production in large scale
(H2020-MSCA-RISE-2016 734708)
3. Exploitation of local diatom diversity for biotechnological applications
IMBBC coordinates the “Centre for the study and sustainable exploitation of Marine Biological Resources (CMBR)” which aims to characterize and exploit marine natural resources for biotechnological applications. Diatoms diversity is unmatched by any other group of microalgae with near 100 00 extent species. Diatom specimens were sampled off Heraklion coast, single cell isolated, placed in laboratory cultures and their (epi)genomic makeup characterized by molecular approaches. In parallel, their nutritional compositions and capacity to produce bioactive metabolites under optimal and stress culture conditions are analyzed (e.g. PUFA profiles and anti-inflammatory bioactive compounds). Promising diatom specimens are selected to further assess their amenability to large scale culturing. We aim with this approach to identify novel diatom specimens with applications in the aquaculture and pharmaceutical industries.
(CMBR-GSRT MIS 5002670)


The 22nd International Conference on Diseases of









